

What is needle blight?
Needle blights are fungal diseases that can affect conifers. Some easily viewed symptoms may appear as chlorosis (yellowing), brown-red spots/bands, and needle necrosis. Multiple years of infection may result in tree mortality.
What is Brown Spot Needle Blight Downloable PDF: Brown Spot Needle Blight
Brown spot needle blight
Lecanosticta acicola is the needle pathogen responsible for brown spot needle blight (BSNB) a disease of several conifer species. This pathogen was first described by F. Thümen (1878) in South Carolina, USA (Tainter & Baker, 1996; Adamson et al., 2018). According to Nest et al., (2019), Mesoamerica (Mexico, Guatemala) is a center of diversity for the genus Lecanosticta but the origin of Lecanosticta acicola is believed to be North America. This fungus is a cosmopolitan pathogen and has been reported in other continents such as Asia (Suto & Ougi, 1998), Africa (Patton, 1997) and Europe (Lavy & Lafaurie, 1994; Cleary et al., 2019). In Europe and Columbia, this fungus is considered as an A2 quarantine pathogen. Conversely, L. acicola has been maintained A1 quarantine status throughout South America, Africa, and Eurasian Economic Union countries (EPPO, 2008).
Brown spot needle blight symptoms are first expressed as yellowish lesions with a clear border sometimes resin-soaked on infected needles. Round black stromata develop under the epidermis of the needle and later form conidiomata. As the infection progresses, lesions increase in size resulting in necrosis and premature defoliation (Adamson et al., 2015). In North America, BSNB infection can take place throughout the year but the highest sporulation of L. acicola occurs in June and August (Kais, 1975).

Lophodermium needle cast Lophodermium is a complex genus comprised of both needle casts pathogens and foliar endophytes on a diverse group of plant hosts (Ortiz-Garcia et al., 2003; Sieber, 1988; Müller & Hallaksela, 1998). In the Rhytismatacea family, the Lophodermium genus is unique for its filiform ascospores and ascocarps that open through a longitudinal slit (Ortiz-Garcia et al., 2003). Over 20 Lophodermium species colonize conifer needles and shrubs (Ortiz-Garcia et al., 2003). Some of these fungi are economically important plant pathogens that cause needle cast diseases in nurseries and plantations.

Lophodermella needle cast There are several species of Lophodermella that can be pathogenic on various species of pine, including, but not limited to, P. elliotii, P. taeda, P. radiata, P mugo, P. contorta, P. sylvestris, and P. nigra (Millar, 1977). Lophodermella needle cast infects the current year’s needles and is known to be a sporadic disease. Once infected the trees take on a scorched appearence. Multiple years of infection can cause severe damage.
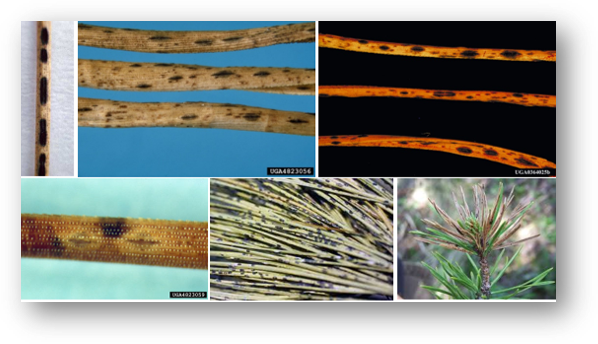
Rhizosphaera needle cast
Rhizosphaera needle cast (RNC), is a foliar disorder of Picea spp. caused by the fungus, Rhizosphaera kalkhoffii (Peace, 1962). This fungus is found in forest plantations, landscapes and natural stands causing needle damage on Colorado blue spruce (P. pungens ), Norway spruce (P. abies) and White spruce (P. glauca). In Japan, the fungus is considered a weak pathogen of P. densflora Siebold & Zucc. especially in areas subjected to drought and SO2 injury (Chiba & Tanaka, 1968). In Europe, the fungus is considered a secondary invader is associated with the top dying of Norway spruce trees, a disease primarily due to water shortage in the crown (Diamandis, 1979). However, in the United States, this is a primary pathogen causing premature needle loss, branch mortality and whole tree defoliation (Blake et al., 1990). A recent study found its association with needle damage of white pine in the New England States and reported it as a secondary invader (Broders et al., 2015).

Dothistroma needle blight Dothistroma septosporum and Dothistroma pini are two distinct fungal species associated with Dothistroma Needle Blight (DNB) also known as red- band needle bight (Barnes et al., 2004). The two Dothistroma needle blight pathogens have a cosmopolitan distribution reported in 76 countries in Eurasia, Oceania, Africa, and the Americas (Drenkhan et al., 2016; Woods et al., 2016). D. pini has spread in central and southern Europe as well as North America and occurs in British Columbia, Indiana, North Dakota, South Dakota, Michigan, Minnesota, and Nebraska (Barnes et al., 2004). The distribution of D. septosporum is wider than D. pini and found in Asia, Africa, North America, South America, Oceania and almost every European country (Drenkhan et al., 2016; Barnes et al., 2004). The pathogen was reported to cause damage on more than 82 different conifer species (Watt et al., 2009). Globally, DNB has devastated many pine plantations across its pine growing region (Barnes et al., 2004). In New Zealand, cost to forest industry due to DNB infection to radiata pines was estimated to be $19.8 million during the first decade of the 21st century (Watt et al., 2011). The fungus enters through stomata and water-soaked lesions start developing on the needles. Black conidiomata surrounded by red band develops at the infection sites and needles become progressively necrotic and cast (Barnes et al., 2004). Infection can cause complete defoliation resulting in stunted growth and mortality (Gibson et al., 1964). The fungus sporulates from April to October and the developing fruiting bodies and sporulation depends on warm and humid weather. Average 15–20°C daily temperature and above 90% relative humidity is optimal for its sporulation, and the fungus can’t sporulate when the temperature is below 180C and relative humidity under 75% (Dvořak et al., 2012). Pine species are highly susceptible to these fungi when they are planted in high rainfall zones. Welsh et al., (2014) reported that August minimum temperature was the most influential climate variable among monthly and seasonally averaged temperature and precipitation variables.

Sydowia needle cast Sydowia polyspora is a foliar endophyte of conifers belonging to Dothideomycetes and is considered a pathogen on several conifer hosts (Pan et al., 2018). This fungus can change its role from endophytic to pathogenic under climatic and biotic stressors (Pan et al., 2018). Sydowia polyspora was found associated with current season needle necrosis and causing needle necrosis and tan to yellow- colored bands after approximately 2-4 weeks of bud break resulted in significant negative impacts on tree growth and marketability of Christmas trees (Talgø et al., 2010). The fungus has a wide geographical range in Europe and North America where it is pathogenic to Thuja, Abies, Tsuga, and Latrix families (Talgø et al., 2010). Current season needle necrosis, a serious foliar disease of Abies spp. has been reported from Europe (Austria, Germany, Norway, and Denmark) and North America (USA).
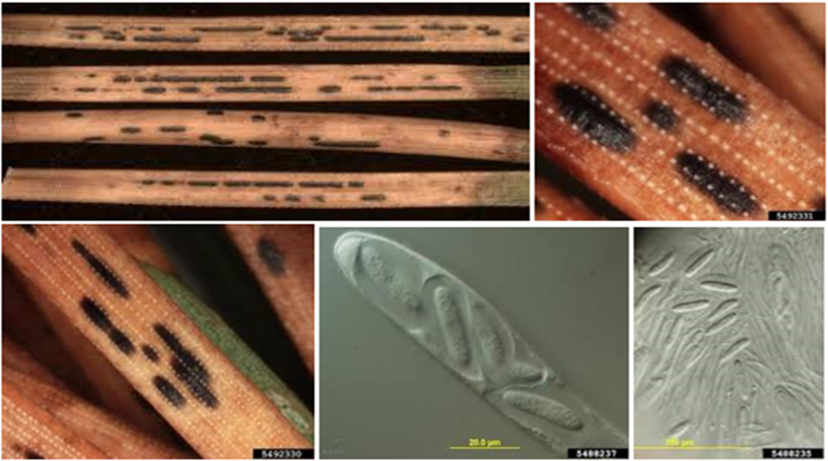
Ploioderma needle cast
Ploioderma needle cast is a needle cast that impacts hard pines (Longnecker and Towers, 1989) although it can be a pest of loblolly and slash pine (Snow, 1984). Following infection afflicted trees might have needles that show a moltted appearance. The next year the needles will shift to a brown or gray on their tips and green on the base. Black elongated bands would run lengthwise along the needle.
![]()
Diplodia tip blight
Diplodia tip blight is caused by the pathogen Diplodia pinea and Diplodia scrobiculata. Diplodia has been documented as an extremely severe pathogen in Austrian, Scots, Ponderosa, and other types of coniferous trees (Brookhouser & Peterson, 1970; Johnson et. al., 1985; Blumenstein et. al., 2021). Diplodia species are well known as opportunistic pathogens that take advantage of tress in weakened or stressed conditions, although healthy trees can become infected. Trees are more likely to be infected during the spring and early summer than in the late summer (Brookhouser and Peterson, 1970). Infections of Diplodia tip blight can cause stunting and curling on needles, necrosis and chlorosis in needle tips on current seasons growth, cankers, branch dieback, and fruiting bodies on needles, shoots, or cones.

Coleosporium needle rust
The genus Coleosporium is composed of rust fungi in the Coleosporiaceae family (Pucciniales, Pucciniomycotina) that includes approximately 100 fungal species that alternate phases between conifers and angiosperms to complete their life cycle (Cummins & Hiratsuka, 2003). This genus is usually macrocyclic and have pycnia, aecia, uredinia, telia and basidia which are subepidermal in the plant tissue. Colesporium asterum has been reported on Solidago in Korea, Canada, and the United States though their morphological characteristics and 28S rDNA sequences are different (Back et al., 2014). Another rust fungus Colesporium solidagin is also found associated with North American Solidago spp. (McTaggart & Aime, 2018). Generally, 2 and 3-needled pines are susceptible to Coleosporium with the pycnia and aecia appearing on Pinus (Weir, 1925) and the uredinial and telial stages appear on woody and herbaceous angiosperms mostly infect members of the Asteraceae (Cummins & Hiratsuka, 2003).

Phytophthora needle diseasePhytophthora is a genus of plant damaging oomycetes and can cause large- scale economic losses in forestry, agriculture, and horticulture (Erwin & Rebeiro, 1996). Over 100 different species have been described species in the Phytophthora genus. Most of them are destructive plant pathogens causing root rots, collar rots, leaf blight and stem cankers (Goodwin, 1997). Phytophthora infections on forest trees were first reported in 1876 in Europe for root and collar rot disease of chestnut trees. Severe mortality of chestnut trees was observed throughout southern Europe due to the spread of P. cambivora Petri Buisman and P. cinnamomi (Crandall et al., 1945; Peace, 1962). Phytophthora cinnamomi, a serious pathogen associated with dying Chestnut trees, was silently spread across the southeastern United States and isolated from 20 different trees species in nurseries ranging from Pennsylvania and Delaware to Louisiana (Crandall et al., 1945). It was once thought that Phytophthora species were not responsible for causing any foliage diseases on pine trees (Williams et al., 2014); however, today conifers are at increased risk for new foliage pathogen. In Chili, a needle blight disease caused by P. pinifolia is locally known as Daño Foliar del Pino (DFP) resulted in rapid death of needles and subsequent defoliation of trees after successive infection. Mature trees to seedlings and needles to stems are susceptible to P. pinifolia (Duran et al., 2008). Similarly, P. pluvialis has caused red needle cast disease of P. radiata resulted in chlorosis, stunted needles, and reduced needle retention (Dick et al., 2014). Similar symptoms have been reported from the Pacific Northwest where the fungus has been isolated from Douglas fir (Brar et al., 2018). About 55 species in the Phytophthora genus has been recognized from 1876 to 1999. Additional 50+ species were recognized and described between 2000 to 2007 (Brasier, 2009).
Click here for a printable document of needle blight images.
References
Adamson, K., Drenkhan, R., & Hanso, M. (2015). Invasive brown spot needle blight caused by Lecanosticta acicola in Estonia. Scandinavian Journal of Forest Research, 30(7): 587-593.
Adamson, K., Laas, M., & Drenkhan, R. (2018). Quarantine pathogen Lecanosticta acicola, observed at its jump from an exotic host to the native scots pine in Estonia. Baltic Forestry, 24(1): 36-41.
Back, C-G., Nam, G-Y., Lee, S-Y., & Jung, H-Y. (2014). Outbreak of rust caused by hi asterum on Solidago virgaurea var. gigantea in Ulleungdo. Mycobiology, 42(1): 79-81.
Barnes, I., Crous, P. W., Wingfield, B. D., Wingfield, M. J. (2004). Multigene phylogenies reveal that red band needle blight of Pinus is caused by two distinct species of Dothistroma, D. septosporum and D. pini. Studies in Mycology, 50: 551–65.
Blake, J. H., Miller, W., Ressler, G., & Boone, Andy (1993). Preliminary Observations of Needle Cast Diseases of Virginia Pine Grown for Christmas Trees in South Carolina. SNA Research Conference, 38: 205-207. Blumenstein K. B., Langer, J., Langer, G.J., and Terhonen, E.J. (2021). The Diplodia Tip Blight Pathogen Sphaeropsis sapinea Is the Most Common Fungus in Scots Pines' Mycobiome, Irrespective of Health Status-A Case Study from Germany. J Fungi (Basel), 7 (8): 607. doi: 10.3390/jof7080607.
Brasier C. M. (2009). Phytophthora biodiversity: how many Phytophthora species are there? In: Goheen EM, Frankel SJ, eds. Phytophthoras in Forests and Natural Ecosystems. Albany, CA, USA: USDA Forest Service: General Technical Report PSW-GTR-221, 101–15.
Broders, K., Munck, I., Wyka, S., Iriarte, G., & Beaudoin, E. (2015). Characterization of Fungal Pathogens Associated with White Pine Needle Damage (WPND) in Northeastern North America. Forests, 6(12), 4088–4104. Brookhouser, L.W. & Peterson, G. W. (1970). Infection of Austrian, Scots, and Ponderosa Pines by Diplodia pinea. Phyto-pathology, 61: 409-414.
Chiba, O., & Tanaka, K. (1968). The effect of sulphur dioxide on the development of pine needle blight caused by Rhizosphaera kalkhoffii Bubak (I). Journal of Japanese Forest Society, 50: 135-139.
Cleary, M., Laas, M., Oskay, F., & Drenkhan, R. (2019). First report of Lecanosticta acicola non‐native Pinus mugo in southern Sweden. Forest Pathology, 49(3).
Cummins, G.B., & Hiratsuka, Y. (2003). Illustrated genera of rust fungi, 3rd ed. APS Press, St. Paul, MN.
Crandall, B.S., Gravatt, G.F., & Ryan, M.M., (1945). Root disease of Castanea species and some coniferous and broadleaf nursery stocks, caused by Phytophthora Cinnamomi. Phytopathology, 3: 162-180.
Dewani, S.A., & Millar, C.S. (1990). Sources of inoculum of Lophodermium seditiosum on Pinus sylvestris. Forest Pathology, 20(1): 1–7.
Diamandis, S. (1979). "Top-dying" of Norway spruce, Picea abies (L.) Karst. with special reference to Rhizosphaera kalkhoffii Bubak. V. Optimum conditions for diameter growth of Rhizosphaera kalkhoffii. European Journal of Forest Pathology, 9: 175-183.
Dick, M.A., Williams, N.M., Bader, M.K., Gardner, J.F., & Bulman, L.S. (2014). Pathogenicity of Phytophthora pluvialis to Pinus radiata and its relation with red needle cast disease in New Zealand. New Zealand Journal of Forestry Science, 44(6).
Durán, A., Gryzenhout, M., Slippers, B., Ahumada, R., Rotella, A., Flores, F., … Wingfield, M. J. (2008). Phytophthora pinifolia sp. nov. associated with a serious needle disease of Pinus radiata in Chile. Plant Pathology, 57(4): 715–727.
J. (2008). Phytophthora pinifolia sp. nov. associated with a serious needle disease of Pinus radiata in Chile. Plant Pathology, 57(4): 715–727.
Dvořak, M., Jankovsky, L., & Drapela, K. (2012). Dothistroma septosporum: spore production and weather conditions. Forest Systems, 21(2): 323-328.
Drenkhan, R., Tomešová-Haataja, V., Fraser, S., Bradshaw, R. E., Vahalik, P., Mullett, M., … Barnes, I. (2016a). Global geographic distribution and host range of Dothistroma species: A comprehensive review. Forest Pathology, 46(5): 408–442.
EPPO (European and Mediterranean Plant Protection Organization). 2005. “ Mycosphaerella dearnessii.” EPPO Bulletin. Wiley Online Library PM 7/46(1). https://doi.org/10.1111/j.1365-2338.2005.00824.x.
Erwin, D. C., & Ribeiro, O. K. (1996). Phytophthora diseases worldwide. St. Paul, Minnesota: APS Press.
Gibson, I. A. S., Christensen, P. S., & Munga, F. M. (1964). First observations in Kenya of a foliage disease of pines caused by Dothistroma pini Hulbary. Commonwealth Forest Review, 43: 31-48.
Goodwin, S.B. (1997). The population genetics of Phytophthora. Phytopathology, 87: 462–73. Johnson, D.W., Peterson, G.W., & Dorset R.D. (1985). Diplodia Tip Blight of Ponderosa Pine in the Black Hills of South Dakota. Plant Disease, 69: 136-137.
Kais, A. G. (1975). Environmental factors affecting brown spot infection on longleaf pine. Phytopathology, 65: 1389–1392.
Levy, A., & Lafaurie, C. (1994). Discovery of Scirrhia acicola. A new foliar pathogen on pine trees attenuata x radiate on the Aquitaine region. Phytoma, 463: 33-35.
Longnecker, J. and Towers, B., 1989. Epidemiology and Control of Ploioderma lethale on Pinus nigra. USDA, Forest Service. General Technical Report WO-56.
McTaggart, A.R., & Aime, M.C. (2018). The species of Coleosporium (Pucciniales) on Solidago in North America. Fungal Biology, 122: 800-809.
Millar, C. S. (1977). Lophodermella species on pines. General Technical Report WO., (50-51).
Müller, M. M., & Hallaksela, A. M. (1998). A chemotaxonomical method based on FAST profiles for the determination of phenotypic diversity of spruce needle endophytic fungi. Mycological Research, 102: 1190-1197.
Ortiz-Garcia, S., Gernandt, D.S., Stone, J.K., Johnston, P.R., Chapela, I.H., Salas-Lizana, R., & Alvarez-Buylla, E.R. (2003). Phylogenetics of Lophodermium from pine. Mycologia, 95: 846–859.
Pan, Y., Ye, H., Lu, J., Chen, P., Zhou, X.-D., Qiao, M., & Yu, Z.-F. (2018). Isolation and identification of Sydowia polyspora and its pathogenicity on Pinus yunnanensis in Southwestern China. Journal of Phytopathology, 166(6): 386–395.
Patton, R.F. (1997). Brown spot needle blight. In: Hansen EM, Lewis KJ, eds. Compendium of conifer diseases. St. Paul, MN: American Phytopathological Society Press. 101 pp.
Peace, T. R. (1962). Rhizosphaera needle-cast. Pathology of Trees and Shrubs with Special Reference to Britain. Clarendon Press, Oxford, England, 315.
Tainter, F. H., & Baker, F. A. (1996). Principles of forest pathology. John Wiley & Sons, Hoboken, NY, 832 pp.
Talgø, V., Chastagner, G., Thomsen, I. M., Cech, T., Riley, K., Lange, K., Klemsdal, S. S., & Stensvand, A. (2010). Sydowia polyspora associated with current season needle necrosis (CSNN) on true fir (Abies spp.). Fungal Biology, 114(7), 545–554.
Sieber, T. N. (1988). Endophytische pilze in nadeln von gesunden und geschädige Fichten (Picea abies (L.) Karsten. European Journal of Forest Pathology, 18: 321–342.
Snow, G. A. 1984. A Needle Blight of Slash and Loblolly Pines in South Mississippi. Recencet Reseacrch on Conifer needle Diseases, Conference Proceedings Gulfport, Mississippi.
Suto, Y., & Ougi, D. (1998). Lecanosticta acicola, causal fungus of brown spot needle blight in Pinus thunbergii, new to Japan. Mycoscience, 39(3): 319–325.
Watt, M. S., Kriticos, D. J., Alcaraz, S., Brown, A. V., & Leriche, A. (2009). The hosts and potential geographic range of Dothistroma needle blight. Forest Ecology Management, 257: 1505–1519.
Watt, M. S., Palmer, D. J., & Bulman, L. S. (2011). Predicting the severity of Dothistroma on Pinus radiata under current climate in New Zealand. Forest Ecology and Management, 261(11): 1792-1798.
Welsh, C., Lewis, K. J., & Woods, A. J. (2014). Regional outbreak dynamics of Dothistroma needle blight linked to weather patterns in British Columbia, Canada. Canadian Journal of Forest Research, 44(3): 212-219.
Weir, J. R., (1925). The Genus of Coleosporium in the north-western United States. Mycologia, 17(6): 225-239.
Williams, N., Hood, I., Dungey, H. S., & Beets, P. N. (2014). Management of red needle cast, caused by Phytophthora pluvialis, a new disease of radiata pine in New Zealand. New Zealand Plant Protection, 67: 48-53.
Woods, A. J., Martín-García, J., Bulman, L., Vasconcelos, M.W., Boberg, J., La Porta, N., … Brown, A. (2016). Dothistroma needle blight, weather and possible climatic triggers for the disease’s recent emergence. Forest Pathology, 46: 443–452.