
Department of Chemistry and Biochemistry
Professor Emeritus
Research Areas: Physical
Office: 267 Chemistry Building
Address: 179 Chemistry Building
Phone: (334) 844-6974
Email: millsge@auburn.edu
Physical Chemistry: Generation of metal and metal-oxide crystallites with nanometer dimensions in nonpolar solvents and investigation of their thermal as well as lubricating properties. Macromolecular photosensitive systems for inactivation of toxins and pathogens with free radicals, adaptive polymer films that experience reversible photochemical transformations for controlling and sensing of chemicals.
Overview
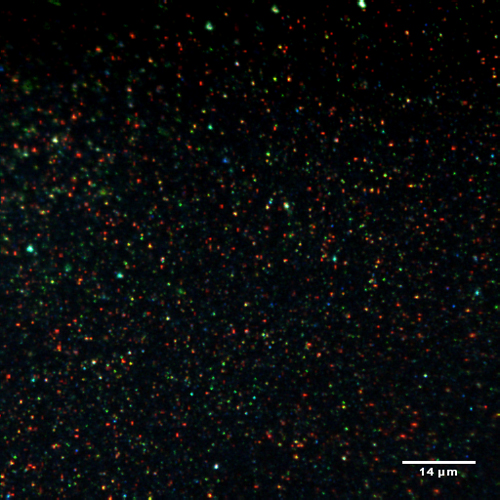
A long-term goal of our work has been the elucidation of the processes leading to formation of solids in liquids, mainly as colloids. An important challenge was the achievement of highly concentrated colloids of nanometer-sized crystallites that remain stable for long times in fluids. Our studies in this area have enabled the preparation of stable colloids containing spherical Ag and CuO particles with concentrations equal or above 0.1 M in liquid polymers and alkanes. Shown in the Figure is an image obtained with a CitoViva optical microscope of Ag crystallites in PEG 600, a liquid poly(ethylene glycol); the various colors originate from crystallites with different sizes. The Ag and CuO colloids are stable ‘nanofluids” exhibiting enhancements in thermal conductivity of about 20% and, more importantly, they possess excellent lubricating properties including low coefficients of friction together with reduced wear. Recent efforts have been centered on the synthesis of concentrated colloids consisting of spherical Ag particles and of rod-shaped CuO crystallites in hydrocarbons. Furthermore, utilization of small SiO2 particles as building blocks for achieving hierarchical structures has led to the reversible formation of transparent silica fibers.
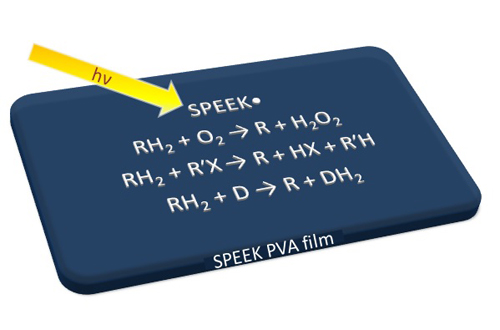
Another area of interest involves exploration of the photochemical properties of macromolecular blends containing sulphonated poly(ether etherketone), SPEEK, and poly(vinyl alcohol), PVA. Benzophenone (BP) groups of SPEEK undergo light-excitation to triplet states that abstract H-atoms from PVA yielding strongly reducing polymeric SPEEK• radicals. Light-exposure of SPEEK/PVA blends in water generated metallic nanometer-sized crystallites. Small metal particles were also formed via illumination of BP in alkanes, providing an alternative method to achieve metallic particles in such difficult-to-work solvents. Our current interest is focused on the ability of SPEEK/PVA films to serve as protective (self-decontaminating) barriers against toxins. Shown in the Figure is a scheme representing the reduction of O2 by SPEEK• to form H2O2, used extensively for the inactivation of microorganisms and toxins. Formation of H2O2 enables the protective function of the films to continue even in the absence of light. Another significant reaction induced by SPEEK• is the degradation of toxic chemicals, an example presented in the Figure is the transformation of the halocarbon R’X. The polymer radical has also been used to transform dyes present in SPEEK/PVA films into forms with different colors, as exemplified in the Figure where D represents a dye. Since the reduced dye is easily re-oxidized, the redox state and the color of the incorporated dyes can be switched in a reversible fashion by photolysis of SPEEK/PVA films.
|
1. Lockhart, P.; Little, B. K.; Slaten, B. L.; Mills, G. “Photogeneration of H2O2 in Water-Swollen SPEEK/PVA Polymer Films” J. Phys. Chem. A 2016, 120, 3867-3877. PDF 2. Little, B. K.; Lockhart, P.; Slaten, B. L.; Mills, G. “Photogeneration of H2O2 in SPEEK/PVA Aqueous Polymer Solutions” J. Phys. Chem. A 2013, 117, 4148-4157. PDF 3. Clary, D. R.; Nabil, M.; Sedeh, M. M.; El-Hasadi, Y.; Mills, G. “Photochemical Generation of Ag, Pd and Pt Particles in Octane” J. Phys. Chem. C 2012, 116, 9243. PDF 4. Clary, D. R.; Mills, G. “Photochemical Generation of Nanometer-Sized Cu Particles in Octane” J. Phys. Chem. C 2011 115, 14656-14663. PDF 5. Clary, D. R.; Mills, G. “Preparation and Thermal Properties of CuO Particles” J. Phys. Chem. C 2011 115, 1767-1775. PDF |
Last updated: 02/13/2025
